Kaisa Helttunen
Assistant Professor in Organic Analytical Chemistry
University of Jyväskylä (opens new window)
Academy Research Fellow, Sep 2017 - Apr 2023
PhD in organic chemistry, University of Jyväskylä 2012
Research interests: supramolecular chemistry, anion binding, macrocyclic receptors (calix[4]pyrroles, resorcinarenes), and colloidal structures including micelles and solid lipid nanoparticles.
Research methods: synthesis, complexation studies (NMR, ITC, UV-vis, fluorescence), and crystallography.
Previous research includes cation complexation, Langmuir films and solid lipid nanoparticles of resorcinarene macrocycles, and anion complexes of aryl amide foldamers.
Contact
kaisa.j.helttunen (a) jyu.fi
Department of Chemistry (opens new window)
Nanoscience Center (opens new window)
P.O. Box 35
FI-40014 University of Jyväskylä
Finland

Macrocyclic Receptors for Selective Anion Extraction
May 2018 - April 2023
The project aims to offer pioneering solutions for the selective binding of biologically and technologically important anions. The target anions include anti-inflammatory drugs with carboxylate groups, and valuable resources, such as phosphate and precious metals (Au, Pt, Co). The project focuses on advanced synthesis of selective receptors, characterization of the anion complexes and development of extraction methods for target anions from water.


Recent Publications
- Małgorzata Pamuła, Evgeny Bulatov, Kaisa Helttunen
Binding of ion pairs and neutral guests by aryl-extended meso‑p-hydroxyphenyl calix[4]pyrrole: The interplay between three binding sites (opens new window)
J. Mol. Struct, 2023, 1273, 134268. doi:10.1016/j.molstruc.2022.134268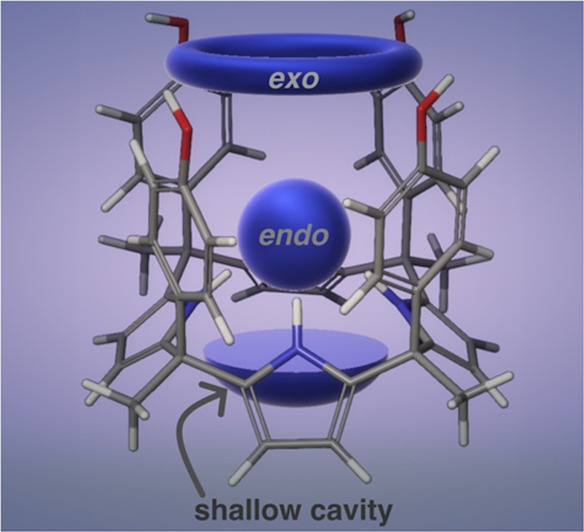 The binding properties of the calix[4]pyrrole host possessing three binding sites were studied by a combination of DFT, NMR, and XRD methods. Binding of acetate, benzoate and chloride anions takes place exo-cavity, while acetonitrile or adiponitrile occupies the deep aromatic endo-cavity leading to crystallization of two-dimensional sheets or dimeric capsules.
The binding properties of the calix[4]pyrrole host possessing three binding sites were studied by a combination of DFT, NMR, and XRD methods. Binding of acetate, benzoate and chloride anions takes place exo-cavity, while acetonitrile or adiponitrile occupies the deep aromatic endo-cavity leading to crystallization of two-dimensional sheets or dimeric capsules. - Kaisa Helttunen
Anion responsive molecular switch based on a doubly strapped calix[4]pyrrole (opens new window)
Eur. J. Org. Chem., 2022, e202200647. doi:10.1002/ejoc.202200647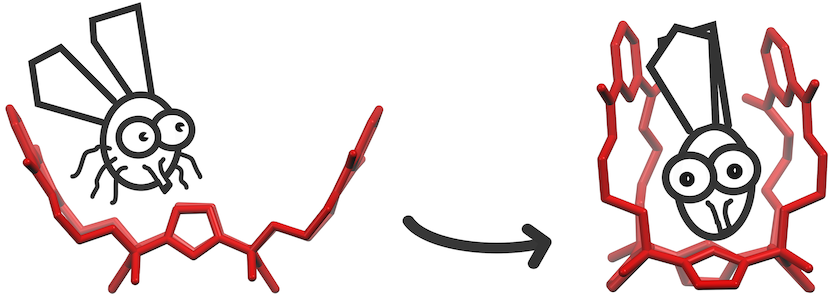 Chloride, acetate and benzoate anions binding with relatively low affinities are kinetically trapped between the straps within the cavity of a doubly strapped calix[4]pyrrole receptor. The complexation is accompanied by a conformational change of the host reminiscent of an insect being captured in a Venus flytrap.
Chloride, acetate and benzoate anions binding with relatively low affinities are kinetically trapped between the straps within the cavity of a doubly strapped calix[4]pyrrole receptor. The complexation is accompanied by a conformational change of the host reminiscent of an insect being captured in a Venus flytrap. - Emilia J. Virtanen, Siiri Perämäki, Kaisa Helttunen, Ari Väisänen, Jani O. Moilanen
Alkyl-substituted aminobis(phosphonates) - efficient precipitating agents for rare earth elements, thorium, and uranium in aqueous solutions (opens new window)
ACS Omega, 2021, 6, 23977-23987. doi:10.1021/acsomega.1c02982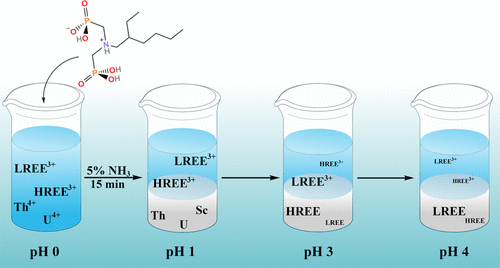 This study reports six simple water-soluble aminobis(phosphonate) ligands, which separate selectively thorium, uranium, and scandium from rare earth elements with improved separation factors in comparison to traditional oxalate precipitation agents. The aminobis(phosphonate) ligands were found to form Y, La and Lu metal complexes with fewer ligands than traditional separation agents like DEHPA, thus requiring less precipitation agent.
This study reports six simple water-soluble aminobis(phosphonate) ligands, which separate selectively thorium, uranium, and scandium from rare earth elements with improved separation factors in comparison to traditional oxalate precipitation agents. The aminobis(phosphonate) ligands were found to form Y, La and Lu metal complexes with fewer ligands than traditional separation agents like DEHPA, thus requiring less precipitation agent. - Małgorzata Pamuła, Maija Nissinen, Kaisa Helttunen
Correlating solution- and solid-state structures of conformationally flexible resorcinarenes: significance of a sulfonyl group in intramolecular self‐inclusion (opens new window)
Chem. Eur. J., 2020, 26, 7374-7383. doi:10.1002/chem.201905211 In this paper we show that a conformationally flexible resorcinarene macrocycle forms a self-inclusion complex in the solid state and adopts a similar folded conformation also in solution. The study was made using VT-NMR, XRD and computational methods.
In this paper we show that a conformationally flexible resorcinarene macrocycle forms a self-inclusion complex in the solid state and adopts a similar folded conformation also in solution. The study was made using VT-NMR, XRD and computational methods. - Kaisa Helttunen, Riia Annala, Aku Suhonen, Juho Iloniemi, Elina Kalenius, Gemma Aragay, Pablo Ballester, Heikki M. Tuononen, Maija Nissinen
Oligoamide foldamers as helical chloride receptors – the influence of electron withdrawing substituents on anion binding interactions (opens new window)
Chem. Asian J., 2019, 14, 647-654. doi:10.1002/asia.201801869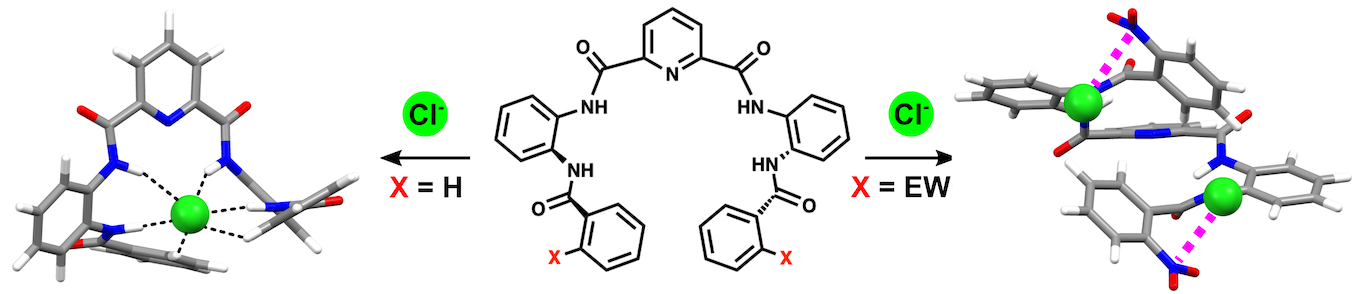 The incorporation of electron‐withdrawing substituents in aryl amide foldamers stabilizes the formation of 1:2 host–guest complexes with chloride anions.
The incorporation of electron‐withdrawing substituents in aryl amide foldamers stabilizes the formation of 1:2 host–guest complexes with chloride anions.
This old page is no longer updated.
Current home page is at https://www.jyu.fi/en/people/kaisa-helttunen (opens new window)